As a credentialed teacher with an M.S. in chemistry, I've noticed that some SAT Chemistry study guides are great, some are so-so, and some must be taken deep into Mordor and cast back into the fiery chasm from whence they came.
Let's start with the great ones:
The Official SAT Subject Test in Chemistry Study Guide
If you can only afford one book, get this one. It has two official practice tests and answer explanations.
Neither of these tests is a copy of the one in The Official Study Guide for ALL SAT Subject Tests, so you should get both books if you can.
Pros
Official material is a true confidence builder. Every question you get wrong contains skills you need to practice.
Most prep books have poorly written questions, answer key errors, and questions that are unrealistically easy, difficult, or off-topic. If you get questions wrong or run out of time on unofficial tests, you'll have trouble figuring out whether the fault lies with you or with the book you're using.
Based on the raw-to-scaled score conversion tables in the book, raw scores of 80/85 and 76/85 will get you perfect 800's on the first and second practice tests,
Cons
There's no Kindle edition, so you'll have to plan ahead and order a physical copy from Amazon.
The Official Study Guide for ALL SAT Subject Tests
This book has an official chemistry practice test that isn't the same as the two in the dedicated chemistry guide (above).
Pros
If you're going to take several Subject Tests, you need this book anyway.
Cons
The test questions in this book are easier than the ones in the dedicated chemistry guide, and the curve reflects that difference: to get a perfect 800, you need a relatively high raw score of 82/85.
Strategy for True/False/CE Questions
- If either the first part or second part of the question is false, don't bubble in CE. For example, neither "All elephants have four legs BECAUSE elephants use their legs to eat peanuts" nor "All elephants have five legs BECAUSE elephants use their legs to walk" deserves the CE mark.
- Mark "CE" if the second part of the sentence is a good reason to believe that the first part is true. For example, "The back side of the moon never faces the Earth BECAUSE scientists have never observed the back side from the Earth's surface" should be marked CE. Strictly speaking, this is not the correct way to use the word because, but it will get you the right answer on SAT Chemistry tests.
Cracking the SAT Chemistry Subject Test
This is a good all-around study guide. It contains content review, useful strategies, and decent practice tests.
Pros
The first two practice tests are very similar to real College Board tests, and there are no answer key errors. The third one, however, contains a few poorly written questions (#104, 37, 38, 56, and 62). You may want to use that one for untimed practice.
The book's helpful content review chapters can keep you from feeling lost. The Chemistry Subject Test covers a broader range of topics than you're likely to learn in your high school class, so content review is a must.
Cons
You'll need a calculator to do some of the practice questions in the content review chapters. You're not allowed a calculator on the actual Subject Test, though, and the full practice tests included in the book are very doable using mental math.
The Princeton Review is all about giving you what you need and not one iota more. Since this book is meant for the Subject Test, you'll need to get an additional study guide if you're planning on taking the AP test, which goes into greater depth and has some additional topics you need to know, such as laboratory chemistry and reaction kinetics.
For the Love of SAT Chemistry (Chris Reddick and Michael Cerro)
This book is geared at about the same level as The Princeton Review's, but it focuses less on textbook-type content review and more on practice problems and answer explanations.
It's an excellent place to start if you like inductive learning. If you prefer to review content in an organized way before starting practice questions, go with The Princeton Review's book.
Pros
The material, including the four practice tests at the back of the book, closely mimics the content and feel of real College Board questions. The answer key is mostly accurate.
Cons
After grading each practice test, you'll be left with a raw score (out of 85 total points) without any way to convert that into a scaled score (out of 800). The scoring instructions and conversion table are waaaaaay back on pages 9-10. Follow the directions carefully: you need to remember to deduct 1/4 of a point for each answer that's incorrect!
Errata from Practice Test 2
#107 is written in an unclear way; don't penalize yourself if your answer doesn't match the book's.
#64 doesn't provide enough information for you to solve the problem, so don't worry if you get this one wrong.
Errata from Practice Test 4
#112's answer should be "T, F, no CE."
#39's answer is correct (C), but since a solution of copper(II) nitrate is blue, not colorless, choice (B) is unnecessarily confusing.
#40's answer is not (C): an ion can't take on a visible color by reflecting ultraviolet wavelengths, which are invisible to the human eye. (D) is a better choice because absorbing more light at some wavelengths than others also suggests that some wavelengths are reflected more than others.
SAT Chemistry Subject Test Problems (Christopher Bozza and Dr. Steve Warner)
This bank of practice questions has the best answer explanations I've seen in any chemistry book. The questions target exactly what's on the Subject Test, and the answer explanations are about two pages long per question.
Pros
This book has the same format as Dr. Warner's SAT and ACT Math books. You can jump right in and start working without having to wade through preliminary reading.
The practice material is very similar to real SAT Chemistry Subject Tests.
The problems in this book are arranged by topic and difficulty level, so students who don't need any content review can jump straight to the chapters that contain what they want to work on.
Cons
Most of the content review is in the answer explanations, so you can't treat this book like a textbook. You really have to engage with the material to receive the maximum benefit.
Errata
#56 on page 81 is worded in an unclear way (and therefore not answerable).
The answer to #110 on page 153 is (B), not (A). The book's answer key is mistaken!
#151 on page 205 is unrealistically difficult. Although you'll need to know how to do unit conversions for SAT Chemistry, you won't have to convert between amperes, coulombs, and moles.
#39 on page 225 has two correct answers: (C) and (E).
#68 on page 233 expects students to (1) figure out that lanthanum has a larger radius than potassium, and (2) go against their intuition that potassium should actually be more reactive, since it's an akali metal that reacts violently with water. Those expectations go against students' experience with metals' reactivities and would not show up on a real test. #71 (below) has a similar problem.
The answer to #71 on page 234 is (B), not (E). Potassium is more metallic than barium based on its Mohs hardness and its reactivity with water.
#83 on page 238 is unrealistically difficult: vapor pressure is related to boiling point, since a liquid boils when its vapor pressure becomes equal to atmospheric pressure. Students shouldn't be expected to know which of the liquids in the list has the highest boiling point (and therefore the lowest vapor pressure). The book's answer is wrong anyway: bromine, octane, and nitrogen trichloride all have boiling points that are higher than 100°C and therefore have lower vapor pressures than water does.
#88 on page 240 is also unrealistically hard. The correct answer should be (C), not (D), since Mg(OH)2 is not a strong base due to its poor solubility in water (0.00064 g/100 mL at room temperature).
#94 on page 241 has two correct answers: (A) and (C).
#125 on page 250 is problematic because the nitrogen atoms in the NH2 groups have lone pairs that can be delocalized into the benzene-like rings through resonance. Those nitrogen atoms are likely to be either sp2-hybridized or somwhere between sp2 and sp3. For this reason, (B) is a better answer than (D).
The second sentence of #130 on page 251 should read "saturated hydrocarbon," not "unsaturated hydrocarbon."
The answer to #136 on page 253 is (B), not (D).
#145 on page 255 has two correct answers, (A) and (B). RbCl and RbF are both soluble in water, while PbO and PbS are insoluble. The soluble salts will produce equal numbers of ions, causing the light bulb to glow with equal intensity, while the insoluble salts will produce negligible concentrations of ions, making the light bulb very dim.
#149 on page 256 is slightly questionable: HI is larger molecule but should also be less polar than HCl, so strictly speaking, students would have to look up the boiling points of both compounds to know the answer. HCl does have a lower boiling point, so it has a higher vapor pressure. (Recall that something boils when its vapor pressure becomes equal to atmospheric pressure, so high-vapor-pressure compounds boil first.)
#159 on page 260 should say, "Absorption of a photon CAN [but doesn't have to] cause electrons to become excited to a higher energy level." Photon absorption can also result in a change in electron spin (radio waves), molecular vibrational states (infrared), or molecular rotational states (microwaves), so the problem needs to be clear that photon absorption can lead to consequences other than just electrons becoming excited (visible and ultraviolet radiation).
Sterling Test Prep SAT Chemistry Practice Questions: High Yield SAT Chemistry Questions with Detailed Explanations
This is a huge bank of practice questions. It's useful if you're already scoring 800 and want to challenge yourself some more.
Pros
Sterling highlights the trickiest topics on SAT Chemistry, including amphoteric compounds, flame test colors, solubility rules, and unusual Lewis structures. If you like hard questions, this is the book to get.
Cons
Despite the claim on the book's cover, most of the questions don't have answer explanations.
Since the questions are organized by topic, you have to work on one chapter at a time. There aren't any timed practice tests.
The book covers some topics that are so hard I doubt they'd ever show up on the Subject Test. For example, it expects you to know the exact role of each of the four quantum numbers. You also have to calculate a dipole moment given the size and distance of two separated charges. (!)
Don't use Sterling until your foundation is very solid. Be willing to Google the explanations for topics you don't understand.
Books to Avoid
I'm not sure how Kaplan's SAT Chemistry (2013-14 edition) got its four-star Amazon reviews. The practice questions in the content review chapters are very calculator-based, and the content review includes some difficult AP-only topics, such as zero, first, and second order reaction kinetics and complicated redox reaction balancing involving H+, OH-, and H2O.
The diagnostic test isn't any better. Out of 85 total questions, two are AP-level rate law questions (#107 and #37), one is an AP-level diffusion rate question (#44), and seven are written in a way that could legitimately make you think there's something wrong with the answer choices (#9, 30, 31, 58-60, and 64). In addition, some of the diagnostic questions are hard to do without a calculator. #69, for example, makes you do a proportion involving the ratio 2/7. (2/7 is about 0.29, in case you're wondering.) Official tests stick to easy fractions like 5/2 or 88/44.
Barron's SAT Chemistry (2009 edition) is even worse: out of 85 questions, the diagnostic test has ten unrealistically tricky questions (#4, 9, 113, 34, 38, 45, 49, 57, 67, and 70) and eight unclearly worded questions (#14, 17, 106, 107, 43, 46, 52, and 56). The 2016 edition fixes questions 4, 17, 113, 45, 52, 56, and 67, but questions 9, 14, 106, 107, 34, 38, 43, 46, 49, 57, and 70 remain problematic.
If you're planning to take the AP test, know that McGraw-Hilll's 5 Steps to a 5 on AP Chemistry (2017 edition) is also really bad. There were so many incorrectly drawn diagrams and poorly written questions on Practice Test #1 alone that I had to quit before I got to the free-response section. I know this book has a four-star Amazon review average, but pay close attention to the negative reviews!
The books above contain everything you need to get an awesome score, but if you'd like personalized help, you can sign up for in-home or online tutoring.
The diagnostic test isn't any better. Out of 85 total questions, two are AP-level rate law questions (#107 and #37), one is an AP-level diffusion rate question (#44), and seven are written in a way that could legitimately make you think there's something wrong with the answer choices (#9, 30, 31, 58-60, and 64). In addition, some of the diagnostic questions are hard to do without a calculator. #69, for example, makes you do a proportion involving the ratio 2/7. (2/7 is about 0.29, in case you're wondering.) Official tests stick to easy fractions like 5/2 or 88/44.
Barron's SAT Chemistry (2009 edition) is even worse: out of 85 questions, the diagnostic test has ten unrealistically tricky questions (#4, 9, 113, 34, 38, 45, 49, 57, 67, and 70) and eight unclearly worded questions (#14, 17, 106, 107, 43, 46, 52, and 56). The 2016 edition fixes questions 4, 17, 113, 45, 52, 56, and 67, but questions 9, 14, 106, 107, 34, 38, 43, 46, 49, 57, and 70 remain problematic.
If you're planning to take the AP test, know that McGraw-Hilll's 5 Steps to a 5 on AP Chemistry (2017 edition) is also really bad. There were so many incorrectly drawn diagrams and poorly written questions on Practice Test #1 alone that I had to quit before I got to the free-response section. I know this book has a four-star Amazon review average, but pay close attention to the negative reviews!
Suggested Study Schedule
Unlike Math Level 2, SAT Chemistry doesn't have many quality prep books. I suggest following the study schedule below.
- Take the first Princeton Review practice test and read the answer explanations.
- Go through all of the content review in the Princeton Review's Cracking the SAT: Chemistry. Alternatively, you can go through the first thirteen chapters of For the Love of SAT Chemistry.
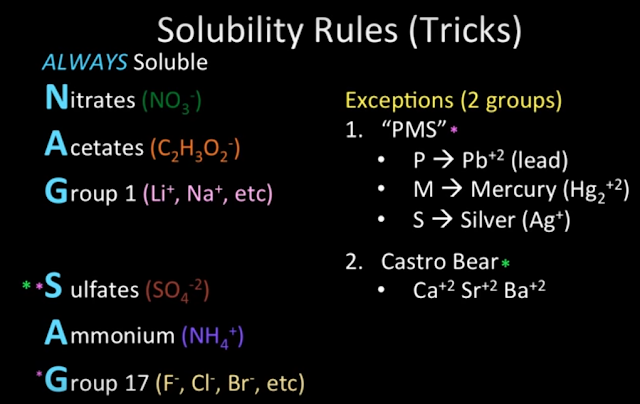
- Read the articles on my Web site about flame test colors, solubility rules, saturation/unsaturation, and avoiding small calculation mistakes.
- Take the second Princeton Review practice test and read the answer explanations.
- Go through Dr. Warner's SAT Chemistry practice book. Read the answer explanations carefully.
- Borrow an AP Chemistry textbook and review your weakest topics. Keep reviewing and re-taking the original two practice tests until your score is 800.
- Take the official practice test in the Official Study Guide for ALL SAT Subject Tests. Go over the test with a fine-toothed comb. Make sure you understand every question so well that you could stand up and teach it in a classroom.
- Do the same for the two official practice tests in The Official SAT Subject Test in Chemistry Study Guide. At this point, you should be scoring solid 800's.
- If you need more practice tests, use the four at the back of For the Love of SAT Chemistry.
- The week before the real test, re-take one practice test a day. Your goal at this point is to increase confidence, not to learn new material. You should receive an 800 on each of the five re-takes. If you don't, you have a good idea of what to review.
Going for a Perfect Score
A raw score of 80/85 will usually get you a perfect scaled 800 on SAT Chemistry. Even after the test deducts a quarter of a point for every question you get wrong, you can afford to miss four of the eighty-five problems. That's like getting a 95% on a comprehensive high school chemistry final.The books above contain everything you need to get an awesome score, but if you'd like personalized help, you can sign up for in-home or online tutoring.