We're back today with the easiest way ever to remember the solubility rules. This three-minute video will save you hours of time with flash cards.
Here's a summary of the mnemonic from the video.
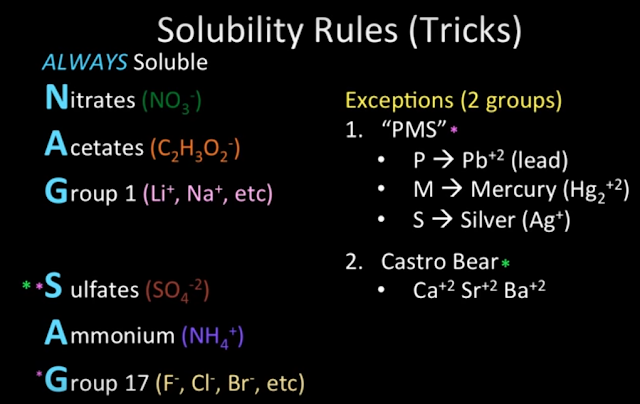
Always Soluble:
- NAG (Nitrates, Acetates, Group 1 alkali metal ions)
- SAG (Sulfates, Ammonium ion, and Group 17 halide ions)
- PMS (Pb2+, Mercury(I) ion, and Silver ions are insoluble when combined with sulfate and group 17 anions.)
- Castro Bear (Ca2+, Sr2+, and Ba2+ are insoluble when combined with the sulfate anion.)
- Note that Castro Bear is still soluble when combined with Group 17 anions. For example, ice melt (calcium chloride) works because it's highly soluble in water, its dissolution is exothermic, and it lowers water's melting point. (Melting/freezing point depression is directly proportional to the number of particles dissolved in a given volume of water. CaCl2 contains three particles per mole and is very soluble, so it lowers water's melting point a lot.)
 |
| No one knew where calcium chloride was at the hardware store. I actually had to ask for ice melt. Imagine that! |
Fun facts
Aqua regia, a mixture of nitric and hydrochloric acids, can actually dissolve gold. The nitric acid oxidizes the gold to Au3+, which then pairs with chloride ions from HCl. Since gold(III) chloride is soluble, the oxidized gold moves into solution, exposing more gold metal to be oxidized. A Jewish scientist used aqua regia to hide two solid gold Nobel Prize medallions from the Nazis.
P.S. Did you know that Fidel Castro actually owned a bear?
 |
| “Fidel Castro with a bear cub Baikal that was given to him by Siberian geologists. The bear went with his new master to Cuba but, unfortunately, could not get accustomed to the local tropic climate.” |










This comment has been removed by a blog administrator.
ReplyDeleteThis comment has been removed by the author.
ReplyDelete